Background: Rabbit Antithymocyte globulin (rATG) is used to prevent graft rejection and graft versus host disease (GVHD) in hematopoietic cell transplantation (HCT). rATG pharmacokinetics are predicted by weight (<40kg) and pre-ATG absolute lymphocyte count (ALC). Using only weight-based dosing results in highly variable post-HCT rATG exposures and impacts outcomes.
Objective: To determine impact of simulated estimated post-HCT rATG exposure on HCT outcomes in recipients of T-replete HCT from a large prospective multicenter database and repository (BMT CTN 1202 study).
Methods: This is a retrospective analysis of data from patients enrolled on BMT CTN 1202 who underwent their first T-replete HCT and received rATG as a part of conditioning. Data from the CIBMTR database and BMT CTN 1202 dataset were used to estimate post-HCT rATG exposures as area under the curve (arbitrary unit per day/milliliter [AU x day/mL]) by using a validated population pharmacokinetic (PK) model (Admiraal et al, 2017). Previously defined post-HCT rATG exposure groups from a single center study: <30, 30-55 and ≥55 AU x day/mL (Lakkaraja et al, Blood advances 2022) were corelated with outcomes of interest: overall survival (OS), CD4+immune reconstitution (CD4+IR) , defined as CD4 + >50/uL by Day+100, transplant-related mortality (TRM), acute GVHD 2-4 (aGVHD), chronic GVHD (cGVHD), and relapse using CIBMTR definitions. Cox proportional hazard and cause-specific hazards models were used for analyses.
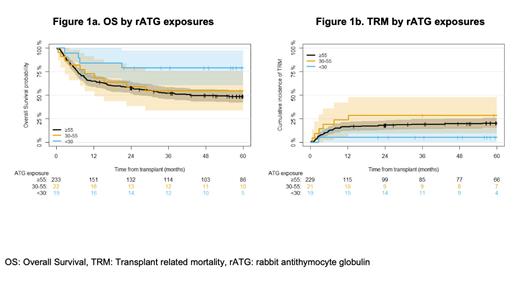
Results: The analysis included 274 patients undergoing T-replete HCT, median age was 46 (range 2 - 73) years, 117 (43%) were female, and 242 (88%) patients underwent HCT for a malignant indication. Myeloablative conditioning was used in 161 (59%) patients and 113 (41%) received non-myeloablative/reduced intensity conditioning. Median post-HCT rATG exposure was 92.2 (range: 0.735 to 677) AU x day/mL. A post-HCT rATG exposure of <30 AU x day/mL was associated with higher OS of 79% at 5 years compared to 55% in the 30-55 AU x day/mL group and 49% in the ≥55 AU x day/mL rATG exposure group (HR for <30 vs ≥55: 0.35, 95% CI: 0.13, 0.95, p=0.05; Figure 1a). Patients with rATG exposure <30 AU x day/mL had a CD4 +IR rate of 86% compared to 44% in the 30-55 AU x day/mL group and 45% in the ≥55 AU x day/mL group (OR <30 vs ≥55: 7.40, 95% CI: 1.20,143, overall p=0.09). Patients with rATG exposure < 30 AU x day/mL had a TRM of 5% at 5 years, versus 29% in the 30-55 AU x day/mL and 20% in the ≥55 AU x day/mL rATG exposure groups (OR <30 vs ≥55: 0.21, 95% CI: 0.03, 1.51, p=0.08; Figure 1b). Post-HCT rATG exposure was not associated with aGVHD, cGVHD, or relapse.
Conclusions: In patients who received a T-replete HCT, a lower post-HCT rATG exposure plays an important role in attaining early CD4+IR and subsequently results in higher OS, likely by lowering TRM. Consistent with our prior work, post-HCT rATG exposures can better predict outcomes suggesting a role for individualized dosing using the validated population PK model; thus, using weight and ALC based dosing compared to weight-based dosing alone. More accurate estimation of the rATG exposure by measuring (active lysing) rATG levels in the available plasma samples and correlation with outcomes in this cohort is ongoing, which we aim to present at ASH.
Disclosures
Levine:Incyte: Consultancy; Bluebird Bio: Consultancy; Genentech: Research Funding; X4 Pharmaceuticals: Consultancy; Viracor: Patents & Royalties: GVHD biomarker patent.; Mesoblast: Research Funding; Incyte: Research Funding; Mesoblast: Consultancy; Inhibrx: Consultancy; Editas: Consultancy; Equillium: Consultancy; Kamada: Consultancy; Sanofi: Consultancy. Perales:Kite: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Adicet: Honoraria; Medigene: Consultancy, Other; Incyte: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria; Syncopation: Honoraria; Cidara Therapeutics: Consultancy, Other; Allovir: Consultancy; BMS: Consultancy, Honoraria; Vor Biopharma: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Sellas Life Sciences: Consultancy; NexImmune: Consultancy, Current equity holder in publicly-traded company; Servier: Other; DSMB: Other; Miltenyi Biotec: Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Merck: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Karyopharm: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Allogene: Research Funding; VectivBio AG: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Boelens:Bluerock: Consultancy, Honoraria; Omeros: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Immusoft: Consultancy, Honoraria; Advanced Clinical: Honoraria; Bluebird Bio: Honoraria; SmartImmune: Consultancy, Honoraria.